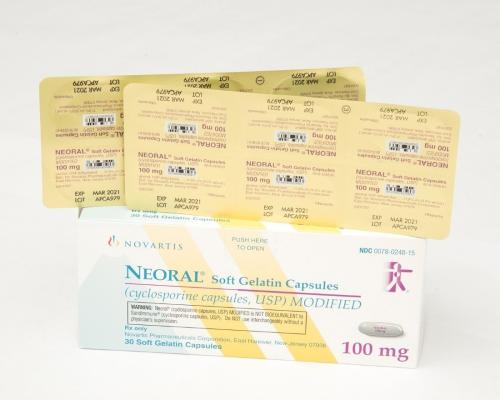
The prescription drug packaging is not child resistant as required by the Poison Prevention Packaging Act (PPPA), posing a risk of poisoning if the contents are swallowed by young children.
Novartis toll-free at 866-629-6182 from 8 a.m. to 8 p.m. ET daily, email at Novartis5060@stericycle.com or online at www.pharma.us.novartis.com and in the top navigation of the page go to the News tab and click on Statements, or visit https://www.pharma.us.novartis.com/news/statements/corrective-action-certain-100-mg-sandimmune-and-neoral-blister-packages-us for more information.
Recall Details
This recall involves blister packages of prescription medications Sandimmune® (cyclosporine capsules, USP) 100 mg soft gelatin capsules and Neoral® (cyclosporine capsules, USP) MODIFIED 100 mg soft gelatin capsules from Novartis. Packages of Sandimmune 100 mg contain three blister cards with ten soft gelatin capsules per card and packages of Neoral 100 mg contain five blister cards with six soft gelatin capsules per card. The recalled blister packages have “Novartis,” the name of the medication, dosage, NDC, lot number and expiration date on the outer package and on the blister cards. Only 100 mg doses of these medications with the following NDC and lot numbers and expiration dates are included in this recall:
|
Recalled Prescription Drugs |
NDC Numbers |
Lot Numbers |
Expiration Date |
|
Sandimmune® (cyclosporine capsules, USP) 100 mg soft gelatin capsules |
0078-0241-15 0078-0241-61 |
APCA136 APCA339 APCA793 APCC238 |
09/2020 02/2021 01/2022 07/2022 |
|
Neoral® (cyclosporine capsules, USP) MODIFIED 100 mg soft gelatin capsules |
0078-0248-15 0078-0248-61 |
APCA437 APCA979 |
07/2020 03/2021 |
Consumers should immediately secure the product out of the sight and reach of children and contact the firm to request a free child-resistant pouch in which to store the blister package medications. Consumers can continue to use the medication as directed. The child-resistant pouches should be used to store these medications until new child-resistant blister packaging is available.
None reported.
Note: Individual Commissioners may have statements related to this topic. Please visit www.cpsc.gov/commissioners to search for statements related to this or other topics.
If you are experiencing issues with a recall remedy or believe a company is being non-responsive to your remedy request, please use this form and explain the situation to CPSC.
The U.S. Consumer Product Safety Commission (CPSC) is charged with protecting the public from unreasonable risk of injury associated with the use of thousands of types of consumer products. Deaths, injuries, and property damage from consumer product-related incidents cost the nation more than $1 trillion annually. Since the CPSC was established more than 50 years ago, it has worked to ensure the safety of consumer products, which has contributed to a decline in injuries associated with these products.
Federal law prohibits any person from selling products subject to a Commission ordered recall or a voluntary recall undertaken in consultation with the CPSC.
For lifesaving information:
- Visit CPSC.gov.
- Sign up to receive our email alerts.
- Follow us on Facebook, Instagram, X, BlueSky, Threads, LinkedIn and Truth Social.
- Report a dangerous product or product-related injury on www.SaferProducts.gov.
- Call CPSC’s Hotline at 800-638-2772 (TTY 800-638-8270).
- Contact a media specialist.