The recalled dietary supplements contain iron which must be in child-resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child-resistant, posing a risk of poisoning if the contents are swallowed by young children.
About 4,000
Nationwide Pharmaceutical at 800-697-3329 from 8 a.m. to 4 p.m. CT Monday through Friday, email at recalls@nwp-mail.com or online at https://nationwidepharmaceutical.com/product-recall/ or https://nationwidepharmaceutical.com and click on “Products” at the top of the page and select “Consumer Information” from the drop down menu for more information.
Recall Details
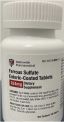
This recall involves Ferrous Sulfate Enteric-Coated Tablets dietary supplements containing 324 mg of ferrous sulfate (iron) in bottles of 100 tablets. “Nationwide Pharmaceutical” and its logo are printed on the top left of the bottle’s label panel. The recalled bottles include lot numbers M0786, M0816, M0817 and M0818, which are printed on the bottom of the bottle.
Consumers should immediately store the recalled dietary supplements in a safe location out of reach and sight of children and contact Nationwide Pharmaceutical for information on how to dispose of the product. Consumers can also return the product to the place of purchase to receive a refund of the purchase price.
None reported
Note: Individual Commissioners may have statements related to this topic. Please visit www.cpsc.gov/commissioners to search for statements related to this or other topics.
The U.S. Consumer Product Safety Commission (CPSC) is charged with protecting the public from unreasonable risk of injury or death associated with the use of thousands of types of consumer products. Deaths, injuries, and property damage from consumer product-related incidents cost the nation more than $1 trillion annually. CPSC's work to ensure the safety of consumer products has contributed to a decline in the rate of injuries associated with consumer products over the past 50 years.
Federal law prohibits any person from selling products subject to a Commission ordered recall or a voluntary recall undertaken in consultation with the CPSC.
- Visit CPSC.gov.
- Sign up to receive our e-mail alerts.
- Follow us on Facebook, Instagram @USCPSC and Twitter @USCPSC.
- Report a dangerous product or product-related injury on www.SaferProducts.gov.
- Call CPSC’s Hotline at 800-638-2772 (TTY 301-595-7054).
- Contact a media specialist.